X chromosome inactivation is a crucial biological process that ensures females, with their two X chromosomes, do not express double the amount of X-linked gene products compared to males. This remarkable mechanism has far-reaching implications for understanding and potentially treating X-linked diseases such as Fragile X syndrome and Rett syndrome. By successfully silencing one X chromosome, cells avoid the detrimental effects of gene overexpression, but it also poses challenges for gene therapy, particularly in cases of genetic disorders. Researchers like Jeannie T. Lee are unlocking the mysteries of this process, revealing how chromosomal therapies might be developed to reactivate the silenced X chromosome and provide relief for patients. This research could ultimately pave the way for innovative treatments for individuals affected by various X-linked diseases, transforming the landscape of genetic disorder management.
The phenomenon of X chromosome inactivation, often essential in the cellular mechanics of females, is a fascinating area of study with potential therapeutic implications. As one of the key strategies employed by female cells to manage gene dosage, it plays a significant role in the silencing of X-linked genes that can contribute to conditions such as Fragile X and Rett syndromes. This intricate silencing mechanism is a vital aspect of chromosomal biology, impacting the development of targeted therapies for genetic disorders associated with the X chromosome. By exploring this complex process, there is hope for advancements in restoring gene function and developing effective interventions for X-linked diseases. As researchers delve deeper, they uncover the potential of chromosomal therapies designed to reactivate beneficial genes that remain suppressed.
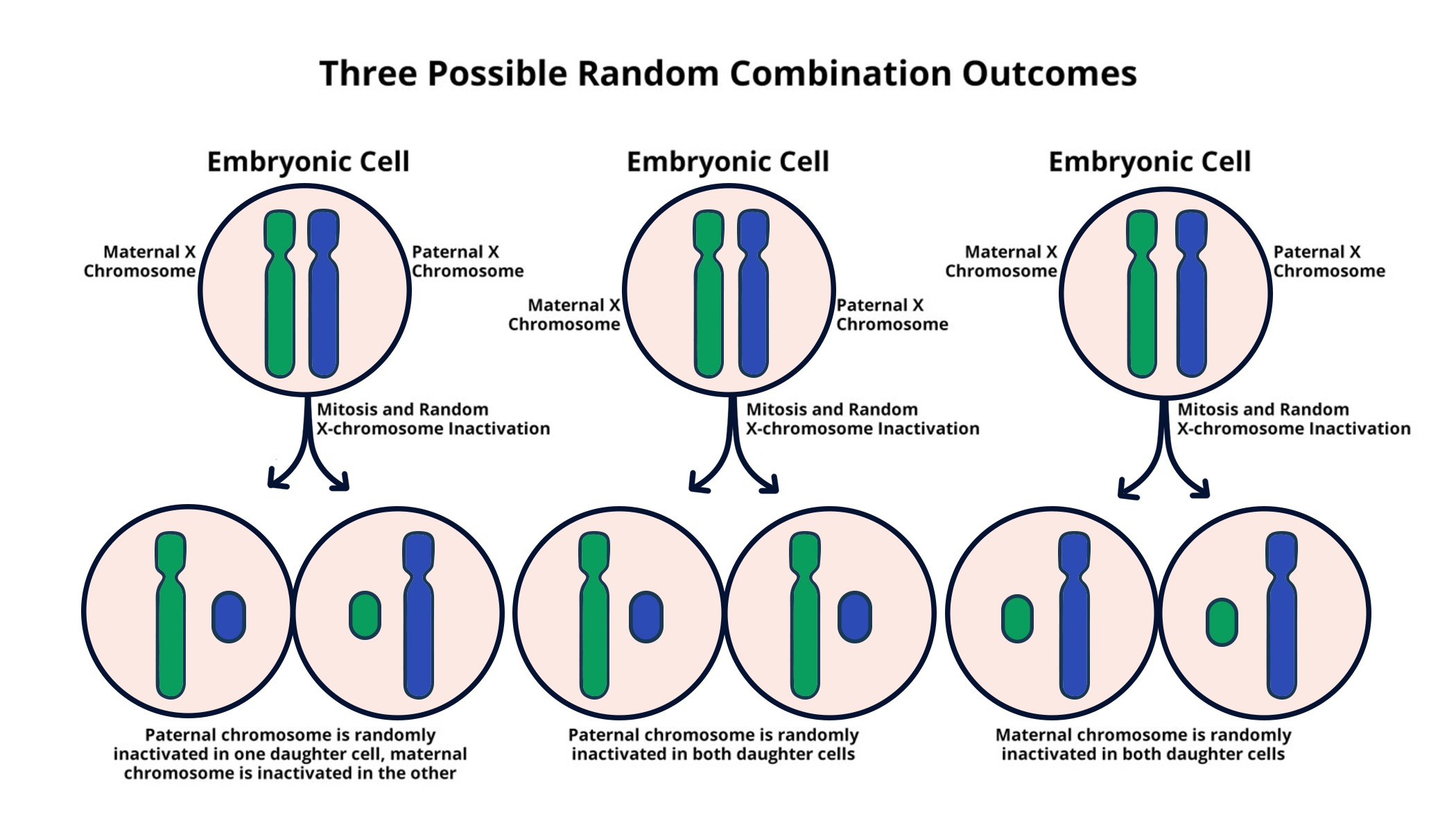
Understanding X Chromosome Inactivation
X chromosome inactivation (XCI) is a pivotal biological process that involves the silencing of one of the two X chromosomes in females. This unique mechanism is crucial because it ensures that the dosage of X-linked genes is balanced between males, who have one X chromosome, and females, who have two. The process is initiated by the expression of the Xist gene, which produces a non-coding RNA that plays a significant role in modifying the chromatin structure of the inactivated X chromosome. This modification process is essential in preventing potential gene dosage imbalance that could lead to developmental issues or diseases.
Recent advancements in understanding XCI have shed light on its broader implications for treating X-linked diseases, such as Fragile X Syndrome and Rett Syndrome. By manipulating the XCI process, scientists hope to develop therapies that can reactivate the silenced X chromosome and allow for the expression of healthy genes. This therapeutic approach not only aims to address existing genetic disorders but also opens the door for potential chromosomal therapies that could correct school-age level developmental disabilities associated with these genetic conditions.
The Role of ‘Chromosomal Jell-O’ in Genetic Therapy
The term ‘chromosomal Jell-O’ refers to the gel-like substance that coats chromosomes, facilitating the distinct organization necessary for normal cellular function. In the context of X chromosome inactivation, this Jell-O-like material interacts with the Xist RNA, allowing for the dynamic process of gene silencing. By elucidating how Xist alters the properties of this gelatinous casing, researchers, including Jeannie Lee’s lab, are exploring potential interventions that could reverse inactivation. This could lead to breakthroughs in the treatment of genetic disorders linked to X chromosome mutations.
These findings are not only important for diagnosing and understanding conditions such as Fragile X Syndrome and Rett Syndrome but also signal significant advancements in chromosomal therapies. The ability to manipulate the ‘Jell-O’ around chromosomes offers potential pathways for reactivating silenced genes, leading to improved treatments for patients affected by X-linked diseases. As research continues in this area, it becomes increasingly clear that understanding the physical properties of chromatin can provide essential insights into developing effective therapies for genetic disorders.
Potential Therapies for Fragile X and Rett Syndromes
Fragile X Syndrome (FXS) and Rett Syndrome are two profound genetic disorders associated with mutations on the X chromosome. FXS often results in cognitive impairments and developmental delays caused by a mutation in the FMR1 gene, whereas Rett Syndrome primarily affects females and leads to severe cognitive impairment and other neurological symptoms. With ongoing research into X chromosome inactivation, exciting therapeutic strategies are emerging to alleviate the symptoms of these challenging conditions. By targeting the pathways involved in XCI, scientists aim to unlock silenced genes and enable the expression of functional variants.
The potential of therapies stemming from our understanding of XCI presents a monumental opportunity for those affected by these X-linked diseases. Early studies have shown that it may be feasible to unsilence the healthy version of genes that were previously inactivated due to mutations. This approach holds the promise of significant improvements in cognitive and behavioral outcomes for individuals with FXS and Rett Syndrome, providing hope for treatment options that tackle the root genetic causes of these disorders.
Advancements in Chromosomal Therapies
The recent findings regarding X chromosome inactivation have spurred interest in the field of chromosomal therapies. These innovative treatment strategies aim to address the underlying genetic mutations by enhancing the expression of silenced genes on the X chromosome. The mechanisms discovered by researchers like Jeannie Lee not only promise to unlock the genes responsible for Fragile X Syndrome and Rett Syndrome but also provide a foundational framework to explore therapies for other genetic disorders linked to the X chromosome.
As researchers refine these chromosomal therapies, the possibility of conducting clinical trials is now on the horizon. Initial studies indicate that freeing inactivated X chromosomes may restore functional gene expression with minimal off-target effects, thereby providing a safer approach to treating genetic disorders. This line of research emphasizes the importance of continued investment in genetic research as it holds the potential to transform lives by making significant strides toward effective treatments for X-linked diseases.
Implications of X Chromosome Research on Genetic Disorders
The research surrounding X chromosome inactivation has far-reaching implications for our understanding of genetic disorders, particularly those that are X-linked. Disorders such as Fragile X Syndrome and Rett Syndrome present significant challenges in diagnosis and treatment, yet the insights gained from studying X chromosome behavior may unlock new pathways for intervention. A deeper comprehension of the mechanisms behind XCI can aid in the development of targeted therapies that allow for the reactivation of beneficial genetic functions, potentially reshaping how these disorders are treated.
Moreover, the exploration of these genetic mechanisms not only contributes to advances in clinical therapies but also enhances our ability to predict the impact of mutations in X-linked genes. This predictive capability is pivotal for developing preventive strategies in at-risk populations and refining therapeutic approaches based on individual genetic profiles, thus promoting a more personalized medicine landscape in the treatment of genetic disorders.
The Future of X Chromosome Research in Medicine
The future of X chromosome research holds tremendous potential for advancing our understanding of genetic medicine. Innovative studies investigating the intricacies of X chromosome inactivation pave the way for breakthroughs that could lead to novel genetic therapies. As researchers continue to explore the mechanisms that govern XCI, the development of therapeutic strategies to reactivate silenced genes could revolutionize treatments for not just Fragile X and Rett Syndromes, but for a wider array of genetic disorders.
Moreover, ongoing advancements in genomic technologies and treatments emphasize the importance of interdisciplinary collaboration in this field. By incorporating findings from genetics, molecular biology, and therapeutic science, researchers are well-positioned to create effective treatments that address the unique challenges posed by X-linked conditions. This collective effort is crucial for transforming research into tangible medical benefits that can profoundly improve the quality of life for individuals affected by genetic disorders.
Exploring Genetic Mutations Linked to X Chromosome
Understanding the variety of genetic mutations linked to the X chromosome is essential for developing targeted therapies for related disorders. X-linked diseases arise from mutations that can disrupt normal gene function, leading to conditions such as Fragile X Syndrome and Rett Syndrome. These mutations can vary in their mechanisms and phenotypic expressions, but they all underscore the critical nature of the X chromosome in human health. By systematically studying these mutations, researchers can identify patterns that may inform the development of specific therapeutic interventions.
Additionally, the role of chromosomal therapies in addressing these genetic mutations comes into sharper focus as we deepen our understanding of X-linked disorders. Focusing on how gene silencing and activation interplay on the X chromosome can lead to innovative methodologies for targeting specific mutations. This not only promises to address the symptoms associated with these disorders but also provides hope for correcting the underlying genetic abnormalities.
The Significance of X Chromosome in Male and Female Genetics
The X chromosome plays a distinct role in female and male genetics, where its implications differ significantly due to the presence of two copies in females and one in males. In females, the mechanism of X chromosome inactivation ensures that one X is silenced, balancing gene dosage between the sexes. Conversely, males express their single X chromosome without such a compensatory mechanism, making them more susceptible to X-linked genetic disorders when mutations occur on their X chromosomes. This fundamental difference highlights the necessity of understanding X chromosome behavior in different sexes for effective treatment strategies.
The effects of mutations can vary widely due to the structural and functional differences in X chromosomes between genders. In males, the presence of a mutation often translates to the manifestation of the disorder without a backup gene, whereas in females, the impact may be masked or modulated by the presence of a second X chromosome. As a result, understanding these dynamics is crucial in devising gender-specific approaches to treatment that consider both the genetic background and the associated intricacies of X-linked diseases.
Current Research Trends in X-linked Genetic Disorders
Current research in X-linked genetic disorders is increasingly focused on unraveling the complex interactions that determine gene expression on the X chromosome. Recent studies emphasize the importance of identifying the specific genetic and epigenetic factors involved in XCI, which could lead to new therapeutic avenues for managing these disorders. Researchers are investigating various genomic editing techniques and RNA-based therapies that have the potential to reactivate silenced genes on the inactivated X chromosome.
Furthermore, the integration of advanced technologies such as CRISPR and gene therapy offers unprecedented opportunities for addressing X-linked diseases. By understanding the underlying genetic mechanisms and leveraging novel therapeutic approaches, scientists aim to develop strategies that can effectively target disorders such as Fragile X Syndrome and Rett Syndrome. This research trajectory promises not only to enhance therapeutic outcomes but also to unlock the potential for early interventions in affected families.
Frequently Asked Questions
What is X chromosome inactivation and how does it relate to Fragile X Syndrome?
X chromosome inactivation is a biological process where one of the two X chromosomes in females is silenced to prevent overexpression of X-linked genes. This process is critical for maintaining balanced gene dosage between males and females. In cases of Fragile X Syndrome, caused by mutations in the FMR1 gene on the X chromosome, understanding X chromosome inactivation may lead to new therapeutic strategies that target the dysfunctional gene.
How does X chromosome inactivation impact Rett Syndrome?
In Rett Syndrome, a neurodevelopmental disorder linked to mutations on the X chromosome, X chromosome inactivation can affect the availability of healthy gene copies. Researchers are exploring ways to unsilence the inactivated X chromosome to potentially restore function to the normal MeCP2 gene, which is crucial for neuronal development and function.
What are some potential therapies being developed for genetic disorders linked to X chromosome inactivation?
Researchers, including Jeannie T. Lee, are developing therapies that aim to reverse X chromosome inactivation in cells with genetic disorders like Fragile X Syndrome and Rett Syndrome. These therapies involve manipulating the processes surrounding the X chromosome, potentially freeing up healthy gene copies that were previously silenced, thus allowing cells to utilize them for normal functioning.
Can chromosomal therapies targeting X chromosome inactivation benefit males with X-linked diseases?
Yes, while males typically have only one X chromosome and do not undergo X chromosome inactivation, therapies targeting the mechanisms of X chromosome inactivation could still benefit males with X-linked diseases like Fragile X Syndrome. These therapies may help in silencing or correcting mutated genes on the X chromosome, thereby improving clinical outcomes.
What role does gel-like substance play in the X chromosome inactivation process?
The gel-like substance surrounding chromosomes, described as ‘chromosomal Jell-O’, is vital for X chromosome inactivation. It helps in organizing the chromatin structure and allows the Xist RNA molecule to alter its biophysical properties. This alteration is essential for initiating the inactivation of one X chromosome, ensuring proper gene dosage and function in females.
| Key Point | Details |
|---|---|
| X Chromosome Inactivation | Females have two X chromosomes while males have one, leading to the need for inactivation of one X in females. |
| Role of Xist | A gene on the X chromosome produces Xist, which modifies the surrounding ‘Jell-O’ substance to facilitate X inactivation. |
| Research Highlight | Jeannie T. Lee’s lab has significantly advanced the understanding of how X chromosome is inactivated. |
| Potential Therapies | Research is paving the way for treatments of Fragile X and Rett syndromes by targeting inactivated X chromosomes. |
| Future Directions | Studies are being conducted to optimize treatments and ensure their safety before clinical trials begin. |
Summary
X chromosome inactivation is a critical biological process, particularly in females where one of the two X chromosomes is rendered inactive to balance gene dosage with males. Recent research led by Jeannie T. Lee has opened up exciting avenues for treating genetic disorders linked to mutations on the X chromosome, such as Fragile X syndrome and Rett syndrome. By understanding and potentially reversing the inactivation of X chromosomes, there is hope for developing targeted therapies that could restore normal function to affected genes. This research not only addresses fundamental questions about gene regulation but also holds promise for significant advancements in genetic medicine.